Forums
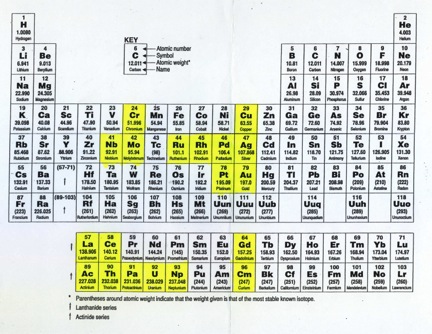
I want to think about the question of the so-called anomalous configurations that occur is some d and f-block atoms (shown in yellow in the diagram below).
- As with the previous posts there is a strong connection with chemistry teaching. This is because students are often taught that the configurations of chromium and copper, in particular, show anomalies from the way that orbitals are occupied as we traverse the transition element series.
- Chromium is said to have a configuration of 3d5 4s1 as opposed to 3d4 4s2. Copper atoms are said to have a configuration of 3d10 4s1 as opposed to 3d9 4s2 as might have been expected from the general trend.
- Now there is good spectroscopic evidence for these anomalous configurations and this is not something I am proposing to question at least not in this post.[1]
- I want to begin to look closely at the commonly given textbook explanation for why chromium has the configuration that it has.
- Textbooks almost invariably claim that the configuration of 3d5 4s1 possesses a half-filled sub-shell and that this is consequently more stable than the expected configuration of 3d4 4s2.
- I think that such an explanation is not very credible but worse, that it is ad hoc in a rather literal sense of the term.
- Of course it may be true that the energy of the anomalous configuration is lower than that of the expected one. After all, why else would the anomalous one be observed if that were not the case?
- But is it because of the much-cited half-filled sub-shell stability? I don’t think so and if you’ll permit me I will replace that mouthful by the abbreviation hfss to stand for half-filled sub-shell from now on.
- To see the limitations of such an explanation we can just look at the atoms in the second transition series that also have anomalous configurations. There are in fact six of them,
- Nb, Mo, Ru, Rh, Pd, Ag
- I am going to ignore palladium and silver for the moment since they have filled d-orbitals and so cannot play any role in arguments considering the role of hfss.
- But of the remaining four cases, only one of them, molybdenum, has a hfss and not surprisingly perhaps it lies directly below chromium in the periodic table.
- Clearly then the adoption of an anomalous cannot be explained in general by appealing to a hfss.
- Here is another way of thinking of this issue by drawing on an approach that is often used in philosophy, and which goes by the name of “necessary & sufficient conditions”.
- Let me illustrate this with an example from chemistry-atomic physics. As is well known, the identity of an element can be established unambiguously by from a knowledge of the atomic number of its atoms. Moreover the relationship runs in the opposite sense too, in that a specification of an atomic number serves to identify the element in question.
- A philosopher looking at this situation might say that the possession of a particular value of Z is both necessary and sufficient for the identification of any element.
- Let me unpack this a little further because such language is not all that familiar in chemistry and physics. If Z = 79, for example, the element must be gold. We say that having an atomic number of 79 is sufficient (is enough) to ensure that the element must be gold.
- Conversely, if the element is to count as gold, it is necessary (essential) for it to have an atomic number of 79.
- The relationship runs in both directions without any exceptions. Because of this fact, we can be confident that the use of atomic number really “nails” the identity of all elements.
- Now let’s go back to those pesky anomalous configurations and the possession or otherwise of ‘hfss’.
- It turns out, rather disappointingly, that the possession of a hfss is neither necessary nor sufficient for an atom to display an anomalous configuration.
- The reason why it is not necessary should be clear from the fact that Nb, Ru, Rh all show anomalies but none of them in fact have a hfss. The reason why it is not sufficient is that there are cases which do have hfss and yet do not show anomalous configurations. This is true of manganese, technetium, rhenium and bohrium all of which have d5 configurations.[2]
- So where does this leave the commonly found explanation for the anomaly in chromium in terms of hfss stability? Simply put – not in a very good place. It so happens that chromium shows both a hfss and an anomalous configuration but this is seldom generalizable.
- In other words, the explanation is ad hoc[3] in the literal sense of having been brought to bear at a particular place (Cr) while being powerless in more general cases.
- So please stop inflicting it on unsuspecting young students!
- Have I labored the point a little? Well perhaps I have but what I am trying to do is to ask chemistry instructors to think a little more deeply and more critically about the contents of chemistry courses and the manner in which material is presented to students.
- Finally, I have said a good deal about how not to explain the configuration in chromium or other anomalous cases but have yet to make any positive suggestions. But that will need to wait for a future blog. There are in fact several better approaches.
Notes
[1] The question of precisely what to regard as the ground state configuration will be taken up in a future posting.
[2] In the f-block Eu and Am have hfss configurations of f7 but don’t show anomalous configurations.
[3] Ad hoc is Latin for “to here” in the sense of an explanation imported into a particular place with little concern for whether it applies in other places or situations. Such an explanation is of course considered to be a ‘bad thing’.
General References
Eric Scerri, A Very Short Introduction to the Periodic Table, Oxford University Press, Oxford, 2011.
For other books please see,